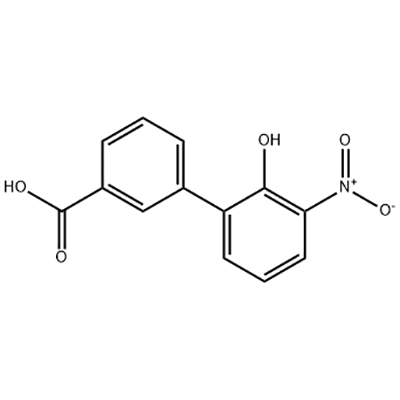
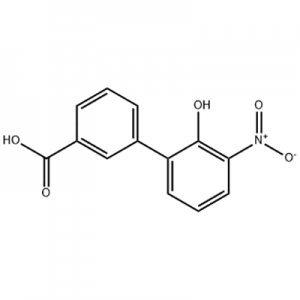
2′-hydroxy-3′-nitro-3-biphenylcarboxylic acid
2′-hydroxy-3′-nitro-3-biphenylcarboxylic acid
2'-hydroxy-3'-nitro-3-biphenylcarboxylic acid waxaa loo isticmaalaa dhexdhexaadka Eltrombopag.
Eltrombopag, oo ay soo saartay GlaxoSmithKline (GSK) ee UK oo markii dambe si wadajir ah ula sameeyay Novartis ee Switzerland, waa kan ugu horreeya oo kaliya ee la ansixiyay molecule yar yar oo aan peptide TPO receptor agonist ee adduunka.Eltrombopag waxaa ansixiyay US FDA 2008 si loogu daweeyo idiopathic thrombocytopenic purpura (ITP), iyo 2014 ee daawaynta aplastic anemia daran (AA).Sidoo kale waa dawadii ugu horeysay ee ay ogolaato FDA US daawaynta AA 30kii sano ee la soo dhaafay.
Bishii Disembar 2012, US FDA waxay ansixisay Eltrombopag daaweynta thrombocytopenia ee bukaannada qaba cagaarshow C dabadheeraad ah (CHC), si bukaannada cagaarshowga C ee qaba saadaasha liidata sababtoo ah tirinta platelet hoose waxay bilaabi karaan oo ay ilaalin karaan interferon ku salaysan daaweynta caadiga ah ee cudurada beerka.Febraayo 3,2014, GlaxoSmithKline waxay ku dhawaaqday in FDA ay siisay shahaadada daawaynta dawaynta ee Eltrombopag ee daawaynta hemopenia ee bukaanada qaba buuga kiimikada daran aplastic anemia (SAA) kuwaas oo aan si buuxda uga jawaabin tallaalka.Agoosto 24, 2015, US FDA waxay ansixisay Eltrombopag daawaynta thrombocytopenia ee dadka waaweyn iyo carruurta da'doodu tahay 1 sano iyo ka weyn ee leh difaaca joogtada ah ee thrombocytopenia (ITP) kuwaas oo aan lahayn jawaab celin ku filan corticosteroids, immunoglobulins ama splenectomy.Janaayo 4, 2018, Eltrombopag waxaa loo ogolaaday in lagu daro Shiinaha si loogu daaweeyo trombocytopenia difaaca aasaasiga ah (ITP).


![Casp ungin Acetate;Caspofungin acetate;Candidas;Caspofungin acetate [USAN:BAN:JAN];](http://cdn.globalso.com/jindunchem-med/fbe17385-300x300.jpg)


![2-butyl-5-nitro-3-benzofuranyl)[4-[3-(dibutylaMino)propoxy]phenyl]](http://cdn.globalso.com/jindunchem-med/922e79ba.jpg)
