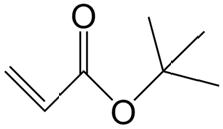
Iibinta kulul ee CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA)
Iibinta kulul ee CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA)
Waxaan si joogto ah u aaminsanahay in qofka dabeecaddiisu ay go'aamiso tayada tayada sare ee alaabta, faahfaahinta ayaa go'aamisa tayada tayada sare leh, oo ay weheliso ruuxa dhabta ah, waxtarka leh iyo hal-abuurka shaqaalaha ee iibinta kulul ee tayada sare leh CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA), Waxaan sameyn doonnaa isku dayo badan oo lagu caawinayo iibsadayaasha gudaha iyo kuwa caalamiga ah, waxaanan dhalin doonnaa faa'iido wadaaga iyo iskaashiga guul-guul ee naga dhexeeya.waxaan si aad ah u sugaynaa wada shaqayntiina si daacad ah.
Waxaan si joogto ah u aaminsanahay in qofka dabeecaddiisu go'aamiso tayada sare ee alaabta, faahfaahinta ayaa go'aamisa tayada tayada sare leh, oo ay weheliso ruuxa shaqaalaha dhabta ah, hufan iyo hal-abuurka lehShiinaha Mipa iyo Monoisopropanolamine, Waxaan isku daraa naqshadeynta, soo saarista iyo dhoofinta si wadajir ah ula in ka badan 100 shaqaale xirfad leh, nidaamka xakamaynta tayada adag iyo technology khibrad leh.We xajiya xiriirka ganacsiga muddada dheer la jumlada iyo qaybiyeyaasha sameeyaan in ka badan dalalka 50, sida USA, UK, Canada, Europe iyo Afrika iwm.
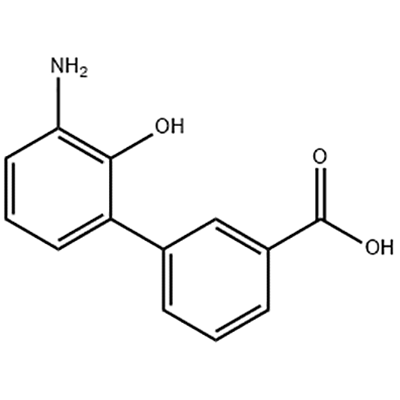
3'-Amino-2'-hydroxy-[1,1'-bipheny]-3-carboxylic acid waxaa loo isticmaalaa dhexdhexaadka Eltrombopag.
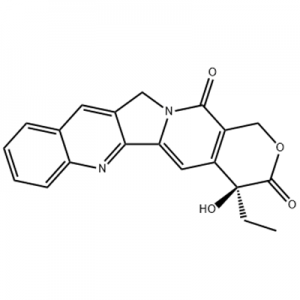
Eltrombopag, oo ay soo saartay GlaxoSmithKline (GSK) ee UK oo markii dambe si wadajir ah ula sameeyay Novartis ee Switzerland, waa kan ugu horreeya oo kaliya ee la ansixiyay molecule yar yar oo aan peptide TPO receptor agonist ee adduunka.Eltrombopag waxaa ansixiyay US FDA 2008 si loogu daweeyo idiopathic thrombocytopenic purpura (ITP), iyo 2014 ee daawaynta aplastic anemia daran (AA).Sidoo kale waa dawadii ugu horeysay ee ay ogolaato FDA US daawaynta AA 30kii sano ee la soo dhaafay.
Bishii Disembar 2012, US FDA waxay ansixisay Eltrombopag daaweynta thrombocytopenia ee bukaannada qaba cagaarshow C dabadheeraad ah (CHC), si bukaannada cagaarshowga C ee qaba saadaasha liidata sababtoo ah tirinta platelet hoose waxay bilaabi karaan oo ay ilaalin karaan interferon ku salaysan daaweynta caadiga ah ee cudurada beerka.Febraayo 3,2014, GlaxoSmithKline waxay ku dhawaaqday in FDA ay siisay shahaadada daawaynta dawaynta ee Eltrombopag ee daawaynta hemopenia ee bukaanada qaba buuga kiimikada daran aplastic anemia (SAA) kuwaas oo aan si buuxda uga jawaabin tallaalka.Agoosto 24, 2015, US FDA waxay ansixisay Eltrombopag daawaynta thrombocytopenia ee dadka waaweyn iyo carruurta da'doodu tahay 1 sano iyo ka weyn ee leh difaaca joogtada ah ee thrombocytopenia (ITP) kuwaas oo aan lahayn jawaab celin ku filan corticosteroids, immunoglobulins ama splenectomy.Janaayo 4, 2018, Eltrombopag waxaa loo ogolaaday in lagu daro Shiinaha si loogu daaweeyo trombocytopenia difaaca aasaasiga ah (ITP).
Waxaan si joogto ah u aaminsanahay in qofka dabeecaddiisu ay go'aamiso tayada tayada sare ee alaabta, faahfaahinta ayaa go'aamisa tayada tayada sare leh, oo ay weheliso ruuxa dhabta ah, waxtarka leh iyo hal-abuurka shaqaalaha ee iibinta kulul ee tayada sare leh CAS 78-96-6 99% Amino-2-Propanol / Isopropanolamine/ Monoisopropanolamine (MIPA), Waxaan sameyn doonnaa isku dayo badan oo lagu caawinayo iibsadayaasha gudaha iyo kuwa caalamiga ah, waxaanan dhalin doonnaa faa'iido wadaaga iyo iskaashiga guul-guul ee naga dhexeeya.waxaan si aad ah u sugaynaa wada shaqayntiina si daacad ah.
Iibinta kululShiinaha Mipa iyo Monoisopropanolamine, Waxaan isku daraa naqshadeynta, soo saarista iyo dhoofinta si wadajir ah ula in ka badan 100 shaqaale xirfad leh, nidaamka xakamaynta tayada adag iyo technology khibrad leh.We xajiya xiriirka ganacsiga muddada dheer la jumlada iyo qaybiyeyaasha sameeyaan in ka badan dalalka 50, sida USA, UK, Canada, Europe iyo Afrika iwm.